Laparoscopic Cholecystectomy
Compared to open cholecystectomy, laparoscopic cholecystectomy requires a longer operative time, but it leads to less pain, a shorter hospitalization, and earlier recovery, and it is associated with a lower morbidity. Cirrhosis is generally considered a relative contraindication for laparoscopic cholecystectomy. Extensive adhesions surrounding the gallbladder make laparoscopic cholecystectomy more difficult. Neither CT nor US can reliably detect these adhesions although MRI may have a role. Nonvisualization of the gallbladder on drip infusion cholangio-graphy appears to have value in predicting extensive adhesions, but this study is rarely performed.
Imaging
Laparoscopic cholecystectomy achieves the same end result as an open cholecystectomy, yet with the former procedure surgeons generally prefer a more specific preoperative diagnosis concerning the presence or absence of bile duct stones; although these stones are readily detected by operative cholangiography, their removal during laparoscopic cholecystectomy is problematic and is one of the causes of conversion to an open cholecystectomy. The luxury of T-tube insertion if bile duct stones are detected and their later removal via the T-tube tract by interventional radiologists is not a viable option during laparoscopic cholecystectomy. Preoperative US aids in predicting whether intraoperative difficulties are encountered. Preoperative US detection of gallbladder wall thickening was found to be the most sensitive and pericholecystic fluid the most specific indicator of a difficult laparoscopic cholecystectomy and the possible need for conversion to laparotomy.
Is ERC necessary prior to routine laparoscopic cholecystectomy? Prior to laparoscopic cholecystectomy, ERC not only detects bile duct stones but also outlines any anomalous biliary anatomy. If stones are found, a sphincterotomy and stone extraction are then performed. Patients having normal biliary US and normal liver function tests have a >95% negative ERC rate, and for these patients preoperatively ERC appears unnecessary. Some surgeons thus argue that endoscopic sphincterotomy prior to laparoscopic cholecystectomy should be reserved only for seriously ill patients or a suspected alignancy, because laparoscopic transcystic duct exploration can be successful in over 90% of cholecystectomies and is safe. Even if laparoscopic cystic duct exploration is not successful, then either open choledochotomy or postoperative endoscopic sphincterotomy is considered.
The role of operative cholangiography during laparoscopic cholecystectomy is not settled and depends, in part, on whether preoperative imaging is performed. Some surgeons obtain it almost routinely, while others use it selectively. The study is safe and adds little to patient morbidity, but it does prolong surgery. A learning curve exists in performing successful intraoperative cholangiography. A success rate of over 90% can be achieved. Digital C-arm fluoroscopy
is a useful guide for intraoperative cholangiography. In general, laparoscopic cholangiography and, if needed, common bile duct exploration obviate a need for a later second procedure such as endoscopic sphincterotomy in patients with retained stones.
Intraoperative cholangiography detects biliary tract complications, and often conversion to an open laparotomy can be performed to repair a visualized injury. Such conversion results in earlier detection of injuries and fewer subsequent procedures to correct the injury. Operative cholangiography performed primarily to detect bile duct stones is discussed later (see Biliary Stones).
Complications
General: Complications occur during laparoscopic cholecystectomy, with postoperative complication rates of 4% to 6% being typical. Initially, after the introduction of laparoscopic cholecystectomy, bile duct injuries were more common than during the previous open cholecystectomy era. Nevertheless, most recent studies conclude that morbidity and mortality of laparoscopic cholecystectomy are lower than after an open operation. The most common complication is a bile leak, followed by retained stones, severe bleeding, subhepatic fluid or abscess, and mild pancreatitis.
Imaging
Laparoscopic cholecystectomy achieves the same end result as an open cholecystectomy, yet with the former procedure surgeons generally prefer a more specific preoperative diagnosis concerning the presence or absence of bile duct stones; although these stones are readily detected by operative cholangiography, their removal during laparoscopic cholecystectomy is problematic and is one of the causes of conversion to an open cholecystectomy. The luxury of T-tube insertion if bile duct stones are detected and their later removal via the T-tube tract by interventional radiologists is not a viable option during laparoscopic cholecystectomy. Preoperative US aids in predicting whether intraoperative difficulties are encountered. Preoperative US detection of gallbladder wall thickening was found to be the most sensitive and pericholecystic fluid the most specific indicator of a difficult laparoscopic cholecystectomy and the possible need for conversion to laparotomy.
Is ERC necessary prior to routine laparoscopic cholecystectomy? Prior to laparoscopic cholecystectomy, ERC not only detects bile duct stones but also outlines any anomalous biliary anatomy. If stones are found, a sphincterotomy and stone extraction are then performed. Patients having normal biliary US and normal liver function tests have a >95% negative ERC rate, and for these patients preoperatively ERC appears unnecessary. Some surgeons thus argue that endoscopic sphincterotomy prior to laparoscopic cholecystectomy should be reserved only for seriously ill patients or a suspected alignancy, because laparoscopic transcystic duct exploration can be successful in over 90% of cholecystectomies and is safe. Even if laparoscopic cystic duct exploration is not successful, then either open choledochotomy or postoperative endoscopic sphincterotomy is considered.
The role of operative cholangiography during laparoscopic cholecystectomy is not settled and depends, in part, on whether preoperative imaging is performed. Some surgeons obtain it almost routinely, while others use it selectively. The study is safe and adds little to patient morbidity, but it does prolong surgery. A learning curve exists in performing successful intraoperative cholangiography. A success rate of over 90% can be achieved. Digital C-arm fluoroscopy
is a useful guide for intraoperative cholangiography. In general, laparoscopic cholangiography and, if needed, common bile duct exploration obviate a need for a later second procedure such as endoscopic sphincterotomy in patients with retained stones.
Intraoperative cholangiography detects biliary tract complications, and often conversion to an open laparotomy can be performed to repair a visualized injury. Such conversion results in earlier detection of injuries and fewer subsequent procedures to correct the injury. Operative cholangiography performed primarily to detect bile duct stones is discussed later (see Biliary Stones).
Complications
General: Complications occur during laparoscopic cholecystectomy, with postoperative complication rates of 4% to 6% being typical. Initially, after the introduction of laparoscopic cholecystectomy, bile duct injuries were more common than during the previous open cholecystectomy era. Nevertheless, most recent studies conclude that morbidity and mortality of laparoscopic cholecystectomy are lower than after an open operation. The most common complication is a bile leak, followed by retained stones, severe bleeding, subhepatic fluid or abscess, and mild pancreatitis.
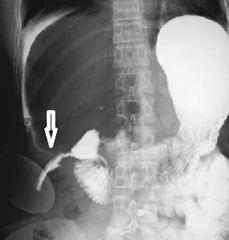
This is radiology images of Duodenal perforation (arrow) detected 2 days after laparoscopic cholecystectomy. A water-soluble contrast agent was used to perform this study.
A common duct stricture and retained stones are late complications, often manifesting as cholangitis rather than jaundice. Because of ease and ready availability, conventional chest and abdomen radiographs should be obtained whenever postlaparoscopic cholecystectomy injury is suspected. They will detect pneumonia and extraluminal gas. Whether they should be followed by CT or US is debatable; either modality detects biliary obstruction and abnormal intraabdominal fluid collections. The definitive study to detect bile duct injury is cholangiography. An ERC can localize a specific site of leakage and detect any underlying strictures. If ERC is unsuccessful or if complete biliary obstruction is encountered, transhepatic cholangiography should define more proximal biliary anatomy. In general, with a major bile duct injury or stricture, percutaneous transhepatic cholangiography is of more value to the surgeon than an endoscopic approach because it defines the proximal biliary tree anatomy used for reconstruction. In a setting of localized disruption, including a cystic duct stump leak or extravasation from ducts of Luschka, placement of a biliary stent is ften therapeutic.
Bile Duct Injury: Most bile duct injuries manifest during the early postoperative period either as obstructive jaundice or a bile leak. Detection of residual stones or a clip in the bile ducts can be delayed.Most complications can be managed successfully by either ERCP or percutaneously. Biliary leaks are successfully treated
by percutaneous biloma drainage combined with either endoscopic or percutaneous transhepatic biliary catheter bypass.
Bile Duct Injury: Most bile duct injuries manifest during the early postoperative period either as obstructive jaundice or a bile leak. Detection of residual stones or a clip in the bile ducts can be delayed.Most complications can be managed successfully by either ERCP or percutaneously. Biliary leaks are successfully treated
by percutaneous biloma drainage combined with either endoscopic or percutaneous transhepatic biliary catheter bypass.
Radiology images of Bismuth classification of bile duct injuries. In type 1, >2cm of hepatic duct is intact; in type 2, <2 cm remains; in type 3, little viable hepatic duct is available; and in type 4, the main right and left lobe ducts are involved.
The Bismuth classification is used to describe major bile duct injuries. A preoperative cholangiogram is valuable prior to bile duct injury repair. Subsequent surgical repair is more difficult and is often unsuccessful if a cholangiogram is not obtained preoperatively or the cholangiogram is incomplete. Common sites for leakage are from a cystic duct stump or from injury to an aberrant bile duct.
This is radiology images of Blown cystic duct stump (arrow). The resultant biloma was drained percutaneously.
Some patients have anomalous small right lobe ducts draining directly into the gallbladder (bile ducts of Luschka), and these are torn during a cholecystectomy.Aberrant bile duct leaks are difficult to detect because these aberrant ducts often do not opacify on operative and postoperative cholangiograms. Bile leakage also occurs with inadvertent bile duct laceration.Most bilomas form around the site of leakage; a rare one extends into the lesser sac. Typically a biloma forms within a week or so after surgery. Some bilomas are associated with jaundice. In general, only the symptomatic patient requires additional therapy. Direct visualization of a leak is by cholangiography— directly via a T-tube, retrograde, or any other access—or indirectly by detecting a biloma with MRCP, CT cholangiography using an intravenous contrast agent, or US. Contrast enhanced MR cholangiography using Mn- DPDP or Gd-EOB-DTPA (agents taken up by hepatocytes and excreted into bile ducts) identifies bile duct leaks in these patients, although advantages of this technique over other methods of visualizing bile ducts are still debated. Ultrasonography reveals a biloma as a sharply marginated anechoic mass having acoustic enhancement. A hematoma or abscess is in the differential diagnosis, although the latter tends to contain a more echoic content. Biliary scintigraphy is probably more sensitive
and more specific than either CT or US in detecting a bile leak.Cholescintigraphy achieves an accuracy of mid-80% in detecting leaks,with CT and US being less sensitive. One can argue that cholescintigraphy should be the first diagnostic modality if a bile leak is suspected but in a number of centers it is relegated to a secondary role. The scintigraphic appearance of a postoperative bile leak is useful for prognosis; if most of the biliary flow is into the duodenum, a perforation will probably resolve without surgical or other intervention.
Occasionally with a small bile leak initial cholescintigraphy is normal, but repeat images obtained after IV morphine reveal a subtle leak. Also, if initial images do not identify a leak, delayed images should be obtained because some small leaks are visualized only several hours later. In spite of these techniques, bile ascites can be difficult to diagnose because of dilution. On the other hand, many small asymptomatic bile leaks are of no clinical significance. Placement of a biliary drainage catheter, inserted either via an endoscopic approach or percutaneously, is sufficient therapy for most localized bile leaks. Endoscopists insert either an intrabiliary stent or a nasobiliary tube across a leakage site, at times adding a sphincterotomy. Endoscopic placement of a short transpapillary stent without a sphincterotomy is an effective and simple way of equalizing pressures within the bile ducts and duodenum. During percutaneous transhepatic biliary drainage, generally performed when surgical or endoscopic therapy is unsuccessful, side holes are positioned on both sides of a leak.
Among postlaparoscopic cholecystectomy patients referred for therapy of bile leaks to members of the Midwest Pancreaticobiliary Group, most common therapy consisted of sphincterotomy with stent insertion, with biliary leakage healing in 88% of patients; percutaneous or surgical drainage of bilomas was required in 32% of patients. With major bile spill into the peritoneal cavity, immediate reexploration is generally indicated. Bile duct transection or other major injury is usually treated with a Roux-en-Y hepaticojejunostomy rather than direct bile duct anastomosis; the latter is associated with subsequent stricture formation, while longterm success rates with a Roux-en-Y hepaticojejunostomy are >80%.Embolization of a biliary leakage site using a percutaneous approach is a potential therapeutic approach.
A clip placed on either the hepatic duct or the common bile duct is the most common cause of acute bile duct obstruction. Obstruction also develops due to inadvertent bile duct cautery or fibrosis for other reasons. Hepatic duct and right hepatic duct necrosis are complications of electrocoagulation. Retained common bile duct stones also result in postoperative obstruction. Some bile duct strictures detected several months after laparoscopic cholecystectomy are associated with a traumatic neuroma, probably induced by prior bile leakage although a thermal injury during cholecystectomy and a resultant fibrous scar may predispose to traumatic neuroma formation. Initially more proximal bile ducts do not dilate after an obstruction, and CT and US may miss a stricture; scintigraphy, on the other hand, will detect an obstruction. In the presence of a bile leak, however, lack of radionuclide activity in the intestines does not imply a more distal bile duct obstruction.
The gold standard for detecting a bile duct obstruction is cholangiography. An MRCP is often a first choice to detect these strictures and any other related complications, such as a leak. Percutaneous or endoscopic stricture dilation is often a viable option; results are comparable to those of surgical reconstruction. With complete obstruction, such as secondary to a clip placed on the hepatic duct, percutaneous drainage of the obstructed ducts is the initial procedure of choice. On the other hand, with a common bile duct stone or cystic duct leak, a sphincterotomy, stone extraction, and an endoprosthesis are generally preferred.
and more specific than either CT or US in detecting a bile leak.Cholescintigraphy achieves an accuracy of mid-80% in detecting leaks,with CT and US being less sensitive. One can argue that cholescintigraphy should be the first diagnostic modality if a bile leak is suspected but in a number of centers it is relegated to a secondary role. The scintigraphic appearance of a postoperative bile leak is useful for prognosis; if most of the biliary flow is into the duodenum, a perforation will probably resolve without surgical or other intervention.
Occasionally with a small bile leak initial cholescintigraphy is normal, but repeat images obtained after IV morphine reveal a subtle leak. Also, if initial images do not identify a leak, delayed images should be obtained because some small leaks are visualized only several hours later. In spite of these techniques, bile ascites can be difficult to diagnose because of dilution. On the other hand, many small asymptomatic bile leaks are of no clinical significance. Placement of a biliary drainage catheter, inserted either via an endoscopic approach or percutaneously, is sufficient therapy for most localized bile leaks. Endoscopists insert either an intrabiliary stent or a nasobiliary tube across a leakage site, at times adding a sphincterotomy. Endoscopic placement of a short transpapillary stent without a sphincterotomy is an effective and simple way of equalizing pressures within the bile ducts and duodenum. During percutaneous transhepatic biliary drainage, generally performed when surgical or endoscopic therapy is unsuccessful, side holes are positioned on both sides of a leak.
Among postlaparoscopic cholecystectomy patients referred for therapy of bile leaks to members of the Midwest Pancreaticobiliary Group, most common therapy consisted of sphincterotomy with stent insertion, with biliary leakage healing in 88% of patients; percutaneous or surgical drainage of bilomas was required in 32% of patients. With major bile spill into the peritoneal cavity, immediate reexploration is generally indicated. Bile duct transection or other major injury is usually treated with a Roux-en-Y hepaticojejunostomy rather than direct bile duct anastomosis; the latter is associated with subsequent stricture formation, while longterm success rates with a Roux-en-Y hepaticojejunostomy are >80%.Embolization of a biliary leakage site using a percutaneous approach is a potential therapeutic approach.
A clip placed on either the hepatic duct or the common bile duct is the most common cause of acute bile duct obstruction. Obstruction also develops due to inadvertent bile duct cautery or fibrosis for other reasons. Hepatic duct and right hepatic duct necrosis are complications of electrocoagulation. Retained common bile duct stones also result in postoperative obstruction. Some bile duct strictures detected several months after laparoscopic cholecystectomy are associated with a traumatic neuroma, probably induced by prior bile leakage although a thermal injury during cholecystectomy and a resultant fibrous scar may predispose to traumatic neuroma formation. Initially more proximal bile ducts do not dilate after an obstruction, and CT and US may miss a stricture; scintigraphy, on the other hand, will detect an obstruction. In the presence of a bile leak, however, lack of radionuclide activity in the intestines does not imply a more distal bile duct obstruction.
The gold standard for detecting a bile duct obstruction is cholangiography. An MRCP is often a first choice to detect these strictures and any other related complications, such as a leak. Percutaneous or endoscopic stricture dilation is often a viable option; results are comparable to those of surgical reconstruction. With complete obstruction, such as secondary to a clip placed on the hepatic duct, percutaneous drainage of the obstructed ducts is the initial procedure of choice. On the other hand, with a common bile duct stone or cystic duct leak, a sphincterotomy, stone extraction, and an endoprosthesis are generally preferred.
This is radiology images of Percutaneous cholangiography in a patient with jaundice after laparoscopic cholecystectomy reveals complete hepatic duct obstruction close to the porta hepatis (arrow). Exploration revealed a metal clip obstructing the hepatic duct.
Cholelithoptysis: Stones are spilled into the peritoneal cavity more often during a laparoscopic cholecystectomy than during an open cholecystectomy.While it was initially believed that no adverse long-term complications follow cholelithoptysis, severe complications requiring a subsequent open surgical procedure have developed. Generally lavage and retrieval of as many stones as possible is attempted after such spill. In fact, open retrieval appears appropriate if several stones or a large stone are lost. Clips have also been spilled into the peritoneal cavity; their long-term consequence is not known.
Some stones eventually become surrounded by granulation tissue. Or gallstones become encased in a pelvic tumor. Some of these patients present months or even years after a cholecystectomy with intraabdominal infection, abscess, or fistula. Gallstones spilled into the peritoneal cavity have led to bowel obstruction. Stones have eroded into the urinary bladder, eroded through the diaphragm, resulted in an empyema, and have even been expectorated. At times the specific etiology for such a calculi-induced abscess is suggested by CT or US. Computed tomography shows a gallstone acting as a nidus for surrounding inflammation. Incomplete Excision: During laparoscopic cholecystectomy the cystic duct is typically transected close to the gallbladder in order to
decrease the risk of hepatic duct and common duct injury. This has led to an incomplete cholecystectomy and eventually recurrent cholelithiasis.A bilobed or duplicate gallbladder is suspected if repeat surgery finds most of the gallbladder still intact in a patient with recurrent symptoms after laparoscopic cholecystectomy.
Some stones eventually become surrounded by granulation tissue. Or gallstones become encased in a pelvic tumor. Some of these patients present months or even years after a cholecystectomy with intraabdominal infection, abscess, or fistula. Gallstones spilled into the peritoneal cavity have led to bowel obstruction. Stones have eroded into the urinary bladder, eroded through the diaphragm, resulted in an empyema, and have even been expectorated. At times the specific etiology for such a calculi-induced abscess is suggested by CT or US. Computed tomography shows a gallstone acting as a nidus for surrounding inflammation. Incomplete Excision: During laparoscopic cholecystectomy the cystic duct is typically transected close to the gallbladder in order to
decrease the risk of hepatic duct and common duct injury. This has led to an incomplete cholecystectomy and eventually recurrent cholelithiasis.A bilobed or duplicate gallbladder is suspected if repeat surgery finds most of the gallbladder still intact in a patient with recurrent symptoms after laparoscopic cholecystectomy.
Imaging studies shortly after a cholecystectomy often detect a pneumoperitoneum. It is of little significance unless it persists. Major infection is not common after laparoscopic cholecystectomy. Computed tomography should identify any abscesses. Arterial trauma during surgery leads to false aneurysm formation; subsequent aneurysm rupture into a bile duct results in hemobilia, at times manifesting months after cholecystectomy. The numerous published reports attest that this is not a rare complication. The right hepatic artery is most often involved, although even a renocaval arteriovenous fistula has been reported. These aneurysms and hepatic artery-to-bile duct fistulas can be successfully embolized.
Unrecognized bleeding due to trocar insertion has resulted in omental or abdominal wall hematomas. A draining umbilical sinus tract has developed at a trocar site. Small bowel has herniated through a trocar site and led to an obstruction. Unsuspected bowel injury may occur. Leakage of intestinal content into the peritoneal cavity induces a peritonitis mimicking bile peritonitis. Small bowel necrosis has been reported. Diaphragmatic injury, even a pneumothorax, has been reported. Underlying primary adrenal insufficiency can lead to shock, bilateral adrenal hemorrhage, and related complications after laparoscopic cholecystectomy.
Unrecognized bleeding due to trocar insertion has resulted in omental or abdominal wall hematomas. A draining umbilical sinus tract has developed at a trocar site. Small bowel has herniated through a trocar site and led to an obstruction. Unsuspected bowel injury may occur. Leakage of intestinal content into the peritoneal cavity induces a peritonitis mimicking bile peritonitis. Small bowel necrosis has been reported. Diaphragmatic injury, even a pneumothorax, has been reported. Underlying primary adrenal insufficiency can lead to shock, bilateral adrenal hemorrhage, and related complications after laparoscopic cholecystectomy.



Post a Comment for "Laparoscopic Cholecystectomy"